Learning Objectives
Understand the ways that free oxygen can be released to the atmosphere.
Explain banded iron formations and what they signify about oxygen levels in the atmosphere and ocean.
Explain redbeds and what they tell us about atmospheric oxygen levels.
Ince early in Earth’s history, free oxygen (O2) has been released in small amounts from the breakdown of water vapor in the upper atmosphere by the Sun’s ultraviolet radiation. By about 3.5 billion years ago, photosynthetic prokaryotes (especially cyanobacteria) also began releasing oxygen to the atmosphere-ocean system. The production of oxygen by photosynthesizers greatly outweighed its production by chemical dissociation in the atmosphere. Stromatolites and thrombolites became much more numerous in the Proterozoic than they were in the Archean. This is an indication that oxygen reached increasingly higher levels in the atmosphere and ocean during this time.
Another indication that oxygen in the atmosphere increased from negligible levels (perhaps 1% to 2% of present levels) in the Archean to much higher levels in the Proterozoic is the occurrence of certain minerals in strata. Pyrite (or “fool’s gold”), a common mineral composed of iron sulfide (FeS2) in sedimentary rocks, forms under low-oxygen conditions, and when exposed to oxygen, it readily disintegrates (or “rusts”) to iron oxide. Pyrite is rare in surface sediments younger than 2.3 billion years. Pyrite, rather than iron oxide, is present in shallow marine and nonmarine sediments of the Archean and earliest Proterozoic, however. This suggests that there was little free oxygen in the atmosphere or ocean before 2.3 billion years ago.
Banded iron formations (BIFs), which are composed of iron minerals interlayered with silica (Figure
9.5), offer further evidence of oceanic oxygen levels. Most banded irons were deposited between 3.6 and 1.9 billion years ago. A few Neoproterozoic and Phanerozoic examples exist, but they tend to be small in size compared to Archean and Paleoproterozoic examples.
Banded iron formations serve as the world’s major sources of iron ore.
Conditions leading to deposition of banded iron formations are controversial, and they probably did not all form in exactly the same type of environment. Some BIFs are associated with turbidites and apparently formed in deep water, whereas others are associated with glacial tillites and seem to have formed in shallow water. One common theme among BIFs is a relationship with igneous activity. In shallow deposits, vents associated with rift valleys emitted hot fluids that were the likely source of iron and silica, whereas in the deep sea, rift systems and magma plumes at hot spots spewed iron - and silica-rich water into surrounding areas.
Iron in BIF deposits is only weakly oxidized, which means that water injected with volcanic emissions had little oxygen in it. The rarity of BIFs younger than 1.9 billion years suggests that about that time, atmospheric oxygen levels rose sharply, primarily through photosynthetic activity. Oceanic mixing would have carried atmospheric oxygen to areas of deeper water, effectively ending the formation of banded irons except in restricted basins such as failed arms of triple junctions or in areas close to volcanic sources.
Buildup of atmospheric oxygen in the Proterozoic was probably enhanced by the filling of chemical sinks for oxygen such as reduced iron and organic carbon. Once large volumes of iron and carbon had been buried, more of the free oxygen released to the atmosphere through photosynthesis could remain there because of the availability of fewer reactive molecules for bonding. Deposition of iron in BIFs left more oxygen to accumulate in the atmosphere. That was followed up toward the end of the Proterozoic Eon by a negative shift in the carbon-13:carbon-12 ratio preserved in limestones
Amazing Places: A banded iron formation figure 9.5
A banded iron formation (BIF) deposit consists of numerous thin layers of iron minerals and silica. Most BIF deposits formed more than 1.9 billion years ago, during the time before the ocean and atmosphere became well oxygenated. Others formed in association with active tectonics and magmatism.
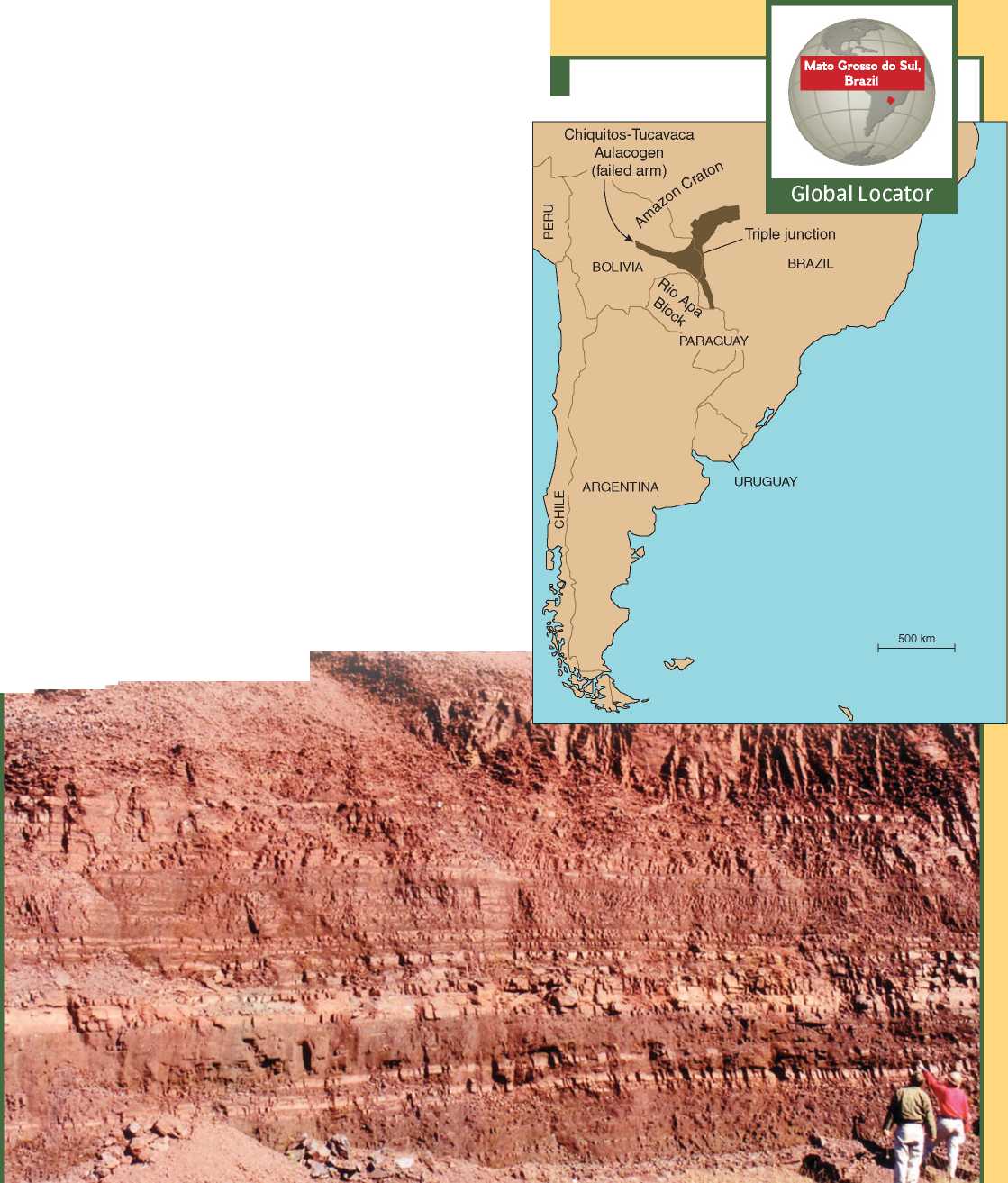
A During the Neoproterozoic Era, in the area that now forms the > border between Brazil and Bolivia, a BIF deposit formed in the failed arm of a triple junction. While glacial ice covered much of the Amazon craton, flexure and extension of the craton caused the downdropping of blocks to form a series of small grabens similar to those forming today in the Great Rift Valley of East Africa. In the Chiquitos-Tucavaca Aulacogen (the failed arm), hydrothermal activity released iron, manganese, silica, and other ions that resulted in the deposition of numerous thin layers of iron minerals and silica. Some manganese layers were also deposited.
B Bands of reddish-brown iron minerals (hematite and magnetite) and black layers of silica (chert) comprising part of the Jacadigo Group (Neoproterozoic) are well exposed in an iron mine in western Brazil. T , .
Oxygenation of the Atmosphere-Ocean System 261
Redbeds, ancient and modern fiqure 9.6

Redbeds, which owe their distinctive reddish color to oxidized iron-bearing minerals, first appeared in the stratigraphic record about 1.9 billion years ago, when the atmosphere became rich in free oxygen. In this scene from South Australia, cliffs of upturned Neoproterozoic redbeds rise above a spring that serves as a watering hole for desert animals. Clay minerals containing iron make up much of the soil surrounding the watering hole. Oxidation of the iron contributes to the reddish-brown color of the Holocene deposit.
Redbeds, which contain well-oxidized, iron-bearing sediments, show a clear relationship to atmospheric oxygenation. The characteristic reddish or reddish-brown color is imparted by oxidized forms of iron such as hematite and limonite (figure
9.6). These minerals form either through oxidation of iron minerals at the time the sediments accumulate (red soils are a common feature of warm areas of the world) or through the secondary oxidation of iron minerals (such as magnetite or pyrite) in sediment. The occurrence of redbeds has an inverse relationship to banded iron formations: they are almost nonexistent in strata older than 2 billion years, and they are prevalent in strata younger than 1.9 billion years. The changeover from BIFs to redbeds marks the time when oxygen levels of the atmosphere and ocean approached levels of the early Phanerozoic. By the Proterozoic-Paleozoic transition, oxygen levels were high enough to support multicellular animals that depended on oxygen for their respiration.
CONCEPT CHECK
In what ways can free oxygen be released to the
Atmosphere? How was free oxygen produced during the Archean and Proterozoic eons?
What are banded iron formations? What do they tell us about atmospheric-oceanic oxygen levels?
What are redbeds? What do they tell us about atmospheric oxygen levels? When did they begin forming?




 World History
World History









