No single analytical protocol has yet been agreed upon as consistently effective in detecting and identifying blood residues. Researchers therefore employ various techniques during excavation, post-excavation, screening, and biochemical analysis of artifacts, according to personal preference and experience. Some investigations of potential blood residues omit certain elements of the investigation process, such as special handling of artifacts with potential blood residues during and after excavation, or the documentation of visible residues prior to extraction.
Examination and Documentation
The first step in residue analysis should involve the examination of the artifact under low-power magnification to locate any possible blood residues. If any
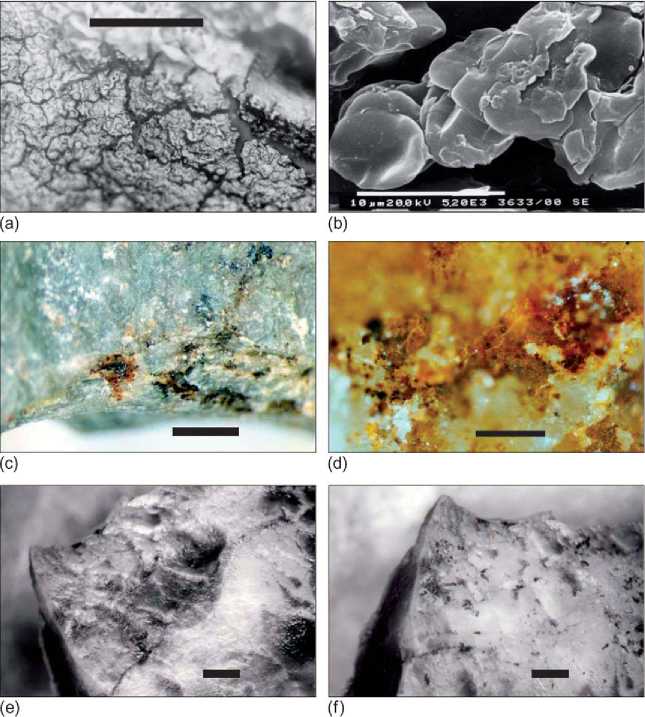
Figure 1 (a), Blood residue on a silcrete stone artifact used to butcher kangaroo (M. fuliginosus, scale bar = 100 mm). (b) Extraction from the same tool in A, visualized in a scanning electron microscope showing individual red blood cells (scale bar = 10 mm). (c) Putative blood residue (dark films) associated with step fractures adjacent to the used edge of a flaked stone artifact from Cuddie Springs, in sediments dated to 30ka. (d) High-power view of residues on flaked stone artifact shown in (c) (scale bar = 100 mm). (e) Surface of experimental tool with blood residue from butchering kangaroo prior to extraction using water as a solvent (scale bar = 1 mm). (f) Surface of same experimental tool shown in (e), showing the incomplete removal of residues (scale bar = 1 mm). Thesurfaceof the tool was lightly scraped with a nylon disposable pipette tip before the solvent plus sample was withdrawn.
Residues are detected, they should be described and photographed. Blood has often been reported as having a distinctive morphology. Dried blood accumulated on experimental tools used to butcher animals produces distinctive residues, straw yellow to dark red in color, exhibiting extensive hexagonal cracking in places (Figure 1a). Scanning electron microscopy of these residues shows intact red blood cells within the yellow-red matrix (Figure 1b).
Red blood cells, if preserved intact, are large enough to be visually identified by light microscopy on lithic tool surfaces. Because the anucleate, concave shape of mammalian red blood cells differs from those of other vertebrates, some researchers have postulated that broad taxonomic distinctions may be made from the observed three-dimensional form of preserved residues. The diameter of red blood cells also varies between species, providing another, more precise prospective diagnostic tool. However, changes in red blood cell size and morphology have been observed during drying of experimental residues, calling into question the potential efficacy of these taxonomic indicators.
Prior to extraction, the artifact may be further tested for the possible presence of blood using a clinical haemoglobin detector, such as Ames Hemastix. Such tests cannot unequivocally identify haemoglobin on artifacts since they have been found to cross-react with non-blood-derived fluids and to produce falsepositive results when exposed to certain elements commonly present in sediment. Blocking cross-reactions may be attempted (e. g., treatment with 0.5 M EDTA to prevent manganese cross-reaction), but cannot be guaranteed. Nevertheless, they can be included as one useful step in the screening process for blood residues, with a positive result indicating that further investigation is necessary.
Extraction and Analysis
Protocols for the recovery of residues from an artifact surface involve either the targeted isolation of visually identified residues (Figure 1e, f) or bulk, whole-tool extractions. There is great variability in extraction methods for putative blood residues and the solvent used is critical to success. The use of 4 M guanidine hydrochloride and 4% ammonium hydroxide rather than water or buffer solutions has been reported to greatly improve recovery and detection rates.
Once extracted from the artifact surface, the residue may be subjected to one or a number of immunological, electrophoretic, and microscopic analyses to determine the presence of blood-derived compounds. Immunological assays, including enzyme-linked immunosorbent assay (ELISA) and Western blot techniques, seek to detect and identify particular proteins derived from blood. In theory, immunological methods can identify specific proteins derived from a particular species, allowing for species-specific identification of blood residues. However, cross-reactivity between species still occurs in practice, reducing the reliable application of immunological techniques to the general detection of (mammalian) blood.
Blood proteins can also be isolated and identified using gel electrophoresis, which uses an electric current to separate molecules by their size. Isoelectric focusing (IEF) involves the separation of the individual proteins in a residue into discrete components by their ionic charge through a pH gradient. Blood residue studies have also attempted to use simple protein assays such as dot-blot and bicinchoninic acid assays.
Often a laboratory will preferentially use one extraction and analysis protocol while another will routinely employ a different method. Differing protocols between laboratories may account for the greatly varying reports for the survival and recovery of blood from stone artifacts and for divergent perspectives on blood survival and/or the form in which blood may be detected. Laboratories do not typically engage in inter-laboratory comparison of results or consultation regarding the relative effectiveness of analytical strategies. Blind tests of the recovery and identification of blood residues that have been undertaken between laboratories have generally produced equivocal results.




 World History
World History









