Stratigraphy The science of rock strata, their relative and absolute ages, and the relationships between strata. water flotation The archaeological method of flotation or water separation involves the use of water (sometimes impregnated with chemicals) to recover tiny artifacts such as seeds, charcoal, nut shell fragments, microdebitage, and bone fragments from soil or feature fill deposits.
‘Palaeoethnobotany’ is the study of human relationships with plants in the past, generally as an adjunct or subdiscipline within archaeology. Although the terms ‘archaeobotany’ and ‘palaeoethnobotany’ have slightly different origins, primarily with respect to initial research aims, today palaeoethnobotany and archaeobotany are often seen as interchangeable terms. Palaeobotany is considered a different subdiscipline, studying plants from earlier times not linked to human impact, as in the Jurassic or Cretaceous times.
Although earlier archaeologists had incorporated fortuitous discoveries of plant materials into their interpretation of the past, beginning as far back as the late nineteenth century, modern palaeoethnobo-tany is a product of the late 1960s processual archaeology, which, building on the work of archaeologists such as Julian Steward, emphasized the central role of biology and the environment in the interpretation of the human past (see Processual Archaeology). The processual era of archaeology saw the advent of many of the most basic tools of palaeoethnobotanists, from sampling design through the now-standard technique of water flotation for recovery of charred plant and other tiny materials. While contemporary palaeoethno-botanists often engage with so-called ‘processual’ issues of diet, environment, and ecology, the research agenda has broadened to include many other themes, such as foodways, gender, interpersonal power issues, identity, trade and exchange, political economy, and all the other topics archaeologists generally engage with through analysis of material culture. After all, for most of human history, the majority of ‘material culture’ was constructed from plants, the most diverse and dynamic group of raw materials available. Plants, however, preserve differently in the ground than more durable objects of bone, stone, and ceramic, and foresight as well as specific tools and processes are necessary to reveal them.
While basic sampling and recovery for plant macroremains has become commonplace (see Macroremains Analysis), research has continued to yield new categories and contexts for archaeological plant remains. Macroremains such as formerly indistinguishable lumps of tissue are now identifiable due to extensive botanical comparative work using scanning electron microscopy (SEM); new categories of microremains, such as starch grains (see Starch Grain Analysis), offer exciting new potentials to interpret traditional ‘blind spots’ in archaeobotanical data; microstratigraphy has permitted the observation of plant material in situ, and various isotopic, spectroscopic, chromatographic, chemical, and molecular techniques record the presence of plants no longer visually recognizable in residues, or as traces in human or animal skeletal material (see Chemical Analysis Techniques; Organic Residue Analysis).
Because plant remains can be either difficult to see or completely invisible to the naked eye, specialized sampling and recovery techniques are employed during excavation. Dependent upon the research design, deposits can have bulk soil samples removed from the site and processed by specialized recovery techniques; such samples may be many liters in volume in the case of flotation samples, or a few scant milliliters or grams for pollen, phytolith, and other microscopic material. Generally archaeobotanical analysis succeeds when a systematic, ‘blanket’ sampling strategy of most or all contexts is employed, enriching interpretation when deposits without obvious quantities of plant remains can be contrasted with material that looks dark or ‘organic-rich’ during excavation.
Aside from fortuitous discoveries of preserved material, macrobotanical plant remains (those visible to the naked eye, but identified using microscopy) are generally recovered from archaeological sites via water flotation, less rarely via nested sieving. Various machine designs have been developed, ranging from simple, nonmechanized setups to elaborate machine-driven froth and water-recycling systems. All water flotation machines share the basic essential principle of using water to separate items with a lower effective specific gravity (charred plant remains, the ‘light fraction or residue’) from the nonbuoyant material or ‘heavy fraction or residue’. Separation of the material into size classes may occur at the flotation stage, or later in the laboratory. In extremely arid sites, such as dry caves (where noncharred material may also survive), botanical remains may be recovered simply with fine sieving. In waterlogged sites, chemical flotation, wherein the specific gravity of the liquid used is increased, may be necessary to properly separate the plant component from the other materials in the deposit matrix. Seeds (including seed-like structures such as nuts, endocarps, or caryopses) and carbonized wood are the most common materials found in flotation samples, but other botanical material, including ‘parenchyma’ (root, tuber, or fruit storage tissue), stems, leaves, inflorescence ‘chaff’, fungal structures, fruit rinds, or attachment structures may also be preserved.
Laboratory identification of macrobotanical material is generally performed by trained analysts who work with identification keys and comparative reference material. The charring process distorts some aspects of seed, wood, and parenchymous tissue anatomy, but much often remains, allowing genus - and often species-level identification, particularly of seeds. The distortion produced by charring may also yield contextual information, as tissues will distort differentially under particular firing conditions (open fire, reduced atmosphere, or dependent on the moisture content of the tissue when it was exposed to fire). Most routine work is conducted using stereoscopic binocular dissecting microscopes, although the increased availability of SEM has permitted greater identification of amorphous tissues and more precise examination of morphological features, such as seed testa thickness and other indicators of domestication or other taxonomic significance. A macrobotanial study of a long-term domestication process of the pseudo-cereal Chenopo-dium quinoa is illustrated from the southcentral highlands of Bolivia (Figure 1). After first identifying grains from flotation samples from Chiripa, Bolivia, Bruno then completed an SEM study of the testa thicknesses of these seeds. In that study, she found that testa thickness varied from the thick wild seeds to the thin domesticated one from the beginning of agriculture up to today, although the domesticates did increase through time.
Quantification of plant remains is less codified in comparison with standard zooarchaeological bone quantifications (such as minimum number of individuals (MNI), number of identified specimens (NISP), useable meat, or biomass calculations), in part because the materials recovered are generally more fragmentary, diverse, and represent not only food taxa, but also commensal and weedy taxa, fuels such as wood or dung, tools, architectural debris, and many other nondietary components. Basic density calculations, normalizing plant part counts, or weights to volume of soil are considered standard, as are percentage presence, diversity, and richness measures of flotation samples. Other ratios or analytic transformations are dependent on the research questions of the investigation. For dietary or other food-related interpretation, comparisons of edible portions to processing waste or fuel (such as maize kernels to cobs, grain to chaff, or nutshell to wood) have been used. Attempts to estimate proportional caloric dietary contributions from macrobotanical remains are fraught with difficulty, although some attempts have been made to statistically rank data from flotation samples in an attempt to understand dietary patterns. Applying more multivariate covariance analyses, especially by usage or environmental zone is increasingly opening up the data. Much work, particularly in cultural resource management contexts (see Cultural Resource Management), continues to be published simply as basic appendixes of identified material, although more research is reaching the general archaeological literature, especially with the increasing interest in foodways. Data presentation ranges from simple presence or absence to elaborate graphs and three-dimensional relationships. The most effective macrobotanical results come from research
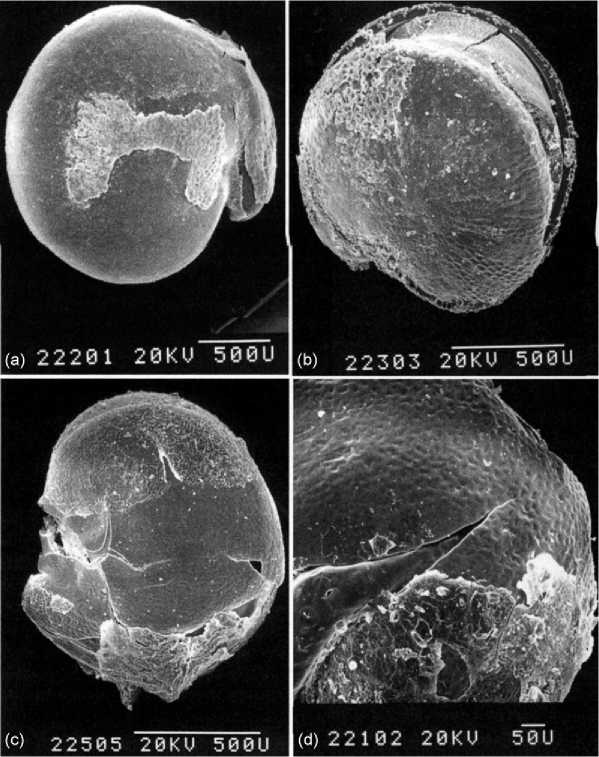
Figure 1 Scanning electron micrographs of experimentally charred modern Chenopodium, showing intact testa and pericarp morphology: a, C. quinoa: the thin testa is intact, and a patch of the pericarp remains in the center; b, C. quinoa var melanospermum: the thick testa is split, and some pericarp remains; c, C. pallidicaule: the testa is intact, and pericarp remains on the margins; d, C. ambrosioides: the testa is intact, and some of the pericarp remains at the bottom of the image. (Reproduced from Bruno M (2006) A morphological approach to documenting the domestication of Chenopodium in the Andes. In: Zeder M, Bradley D, Emshweler E, and Smith BD (eds.) Documenting Domestication, New Genetic and Archaeological Paradigms, pp. 32-45. Berkeley: University of California Press, with permission.)
That thoroughly collected the plant matter, systematically identified the material, and kept the statistical manipulation to a minimum, in line with the intensity of collection. A clear illustration of this is in Johannessen’s research where she demonstrates the long domestication and propagation of indigenous local seeds throughout the Midwestern US, long before maize entered the region, illustrated in Figure 2.
Beginning in the late 1970s, a suite of new so-called ‘microremains’ began to emerge more prominently as part of the archaeobotanical toolkit. Microremains are generally described as plant remains too small to be seen without microscopy, and are usually chemically extracted from soils and sediments. Palynology (see Pollen Analysis) has had a long history of indirect application for archaeologists, tracking ecological and climate change over long periods of time through off-site coring studies, yet renewed attention was paid to the potential of on-site pollen for archaeological interpretation. Phytoliths, plant opal silica particles, were first described as a potential archaeological tool by Rovner in 1971, and the first major interpretive study was by Pearsall in 1979 where she tracked the entry of maize into Ecuador.
Archaeological palynology rests on the interpretation of pollen, the male gametophyte of flowering plants, as well as spores from nonflowering plants and fungi, which have a similar chemical structure. Palynology as a palaeoecological reconstructive technique has been well known since the late nineteenth century, using core samples from highly stratified natural deposits such as varves and other lake sediments. Taxonomic identification of pollen is quite
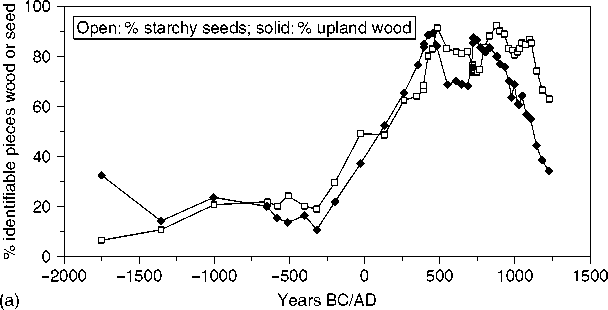
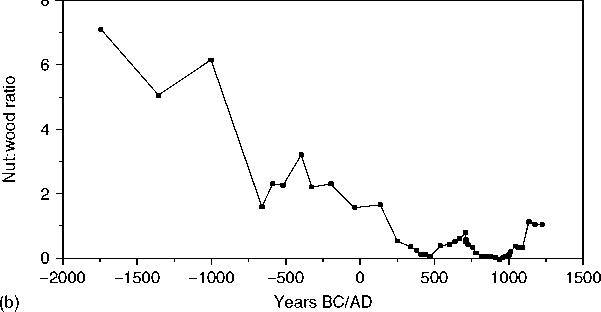
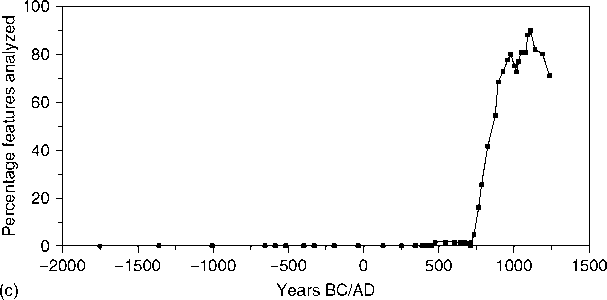
Figure 2 a, Smoothed data for percentage of starchy seeds and percentage of upland wood. b, Smoothed data for nut:wood ratio. c, Smoothed data for percentage of features with maize. (Reproduced from Hastorf CAand Popper VS (1988) Current Paleoethnobotany. Chicago: University of Chicago Press, with permission.)
Secure, often possible to genus or other subfamily levels, although wind-pollinated (anemophilous) species will tend to be overrepresented simply because they produce many more times the amount of pollen than animal - or insect-pollinated species. This discrepancy in production may be valuable to archaeologists, as unusual amounts of nonanemophilous pollen grains may indicate deliberate use of plant taxa. Its success as an on-site tool in archaeological sediments is highly variable; because pollen is omnipresent in
The air, pollen samples are more susceptible to contamination than macrobotanical or phytolith samples. Moreover, the conditions necessary for pollen preservation and the amount of contamination via translocation through soil profiles on open-air sites is the subject of some concern and unresolved questions.
Pollen is chemically extracted from soils and sediments, generally using very small initial volumes of matrix, on the order of a few cubic centimeters. There is some debate over whether the procedures perfected for lake sediments and core samples should be used unmodified on samples from archaeological contexts. Generally, though, strong reagents such as hydrofluoric acid are used to dissolve the mineral fraction, leaving only the organic material, including the polymer sporopollenin, which comprises the exine, or outer structure of fossil pollen grains. Additional organic material such as charcoal particles or cellulose may also be preserved in the pollen extraction procedure; these may provide supplementary contextual information to the analyst.
Pollen is identified using a combination of published keys, atlases, and comparative collections; pollen morphology and taxonomy is well described and understood due to its long history of application. Pollen is generally quantified by a standardized count of several hundred identifiable pollen grains. Usually a precise amount of an exotic ‘tracer’ pollen grain or spore is also added to the pollen sample during the extraction procedure, which provides a way to quantify pollen abundance in a sample. Counts of the exotic type are tallied along with the original grains present in the sample. The ratio of archaeological pollen grains to tracer grains counted during the analysis provides an estimation of the density of pollen preserved in the original sample.
Traditionally, pollen frequency diagrams have been the primary means of presenting and interpreting pollen data; arranged stratigraphically, these charts present the fluctuating relative levels of pollen types through time. These depictions represent a sequence through time.
Phytoliths are rapidly becoming an interpretive boon to archaeological interpretation as they become better understood in terms of formation process, recovery, and interpretive capacity (see Phytolith Analysis). Phytoliths are plant cells or intercellular voids in plant tissue that have accumulated silica from groundwater and solidified. They retain distinct shapes and are taxonomically identifiable to varying levels of specificity. Phytoliths are generally pursued on sites where macrobotanical recovery is sparse, because silica is much less susceptible to destruction than fragile charred plant tissue. Nonetheless, like pollen, they are a complementary rather than substitute data set. Phytolith production is primarily genetically controlled, and many plant families produce either no phytoliths at all, or amorphous or redundant phytolith types that are not taxonomically distinct. Phytolith production will also vary according to various edaphic, environmental, and climatic conditions, where increased evapotranspiration rates may increase silica deposition in tissues. In general, mono-cotyledonous species, particularly the grasses (Poa-ceae family), tend to be heavy silica accumulators and are overrepresented in comparison to dicotyledonous species in phytolith assemblages.
Phytoliths can be recovered from both artifacts such as stone tools and pottery as well as bulk soil samples. Extraction laboratory processes are variable by necessity, as some soils react poorly with individual chemicals used in processing. Generally, though, removal from soil consists of physical or chemical separation of phytoliths from the surrounding soil matrix by some combination of disaggregation, removal of organic materials through combustion or acid digestion, and usually a chemical flotation separating the phytoliths from mineral components of the sediment sample.
Phytoliths are generally quantified using transmitted light microscopy at magnifications of 100-1000 x. Phytoliths fall in the range of 5-500 pm (0.005-0.05 mm), and their translucent, three-dimensional structure requires viewing in multiple orientations for secure identification. SEM is also a productive tool in comparative phytolith studies, but cumbersome for general counting purposes.
The analytical classification of phytoliths has been hampered until recently by the lack of a standardized descriptive terminology. In 2005, the first International Code of Phytolith Nomenclature (ICPN) was published, providing a common language for description of phytolith forms, permitting easier comparison between regions and analysts. Some identification keys and regional publications exist, and specific taxa such as domesticates are well described. However, most analysts must develop a regional comparative collection for their study area. Several researchers have made image and other descriptive databases of comparative collections available online.
Phytolith quantification procedures were largely borrowed from palynological techniques. Standardized counts (i. e., counting a target number of known forms) are the most common quantification, although other methods may be employed. Because phytoliths do not have the same type of specific taxonomic affiliation of pollen, multivariate statistical techniques such as principal components, factor analysis, or correspondence analysis are often used in lieu of the traditional pollen frequency diagram when interpreting multiple phytolith samples, although such diagrams may be useful when phytoliths are recovered from soil cores or other types of column samples. Phytoliths have also been used as material for generating accelerator mass spectroscopy radiocarbon dates, via carbon sometimes occluded in phytoliths.
Starch grains have been the subject of much recent research. They are nutrient storage structures that form in all parts of plants, but are particularly numerous and large in edible roots and tubers, the staples of numerous diets and economies.
Like pollen, they are composed of organic polymers that are durable in many buried environments. Starch grain analysis has great potential to address a traditional ‘blind spot’ in archaeobotancial analysis, namely horticultural root and tuber crops, which preserve poorly as macroremains and are common staples in tropical and subtropical regions where general preservation conditions are also poor.
Extraction procedures and taxonomies are at the earliest stages of development, although research is progressing quickly. Starch grains are classified by size, shape, surface decoration, and the shape of the ‘extinction cross’ produced by the crystalline structure of the grains when viewed in cross-polarized light. However, it is difficult to interpret starch grains recovered from generalized contexts such as soil samples, as too little is known about life-cycle production across a range of plant types. Starch grain analysis has proved most fruitful in specific contexts such as residue studies of food preparation artifacts, where a limited number of possible starchy plants may be distinguished from one another.
Numerous other high-powered techniques in archaeology are shedding light on archaeobotanical issues such as stable isotopes, organic residues, and lipid analysis, providing further evidence of plant use in the past by humans. These techniques used together increase our potential to visualize past use of plants.
See also: Archaeology Laboratory, Overview; Blood Residue Analysis; Bone Tool Analysis; Cultural Resource Management; DNA: Ancient; Ecofacts, Overview; Insect Analysis; InterdisciplinaryResearch; Invertebrate Analysis; Macroremains Analysis; Organic Residue Analysis; Phytolith Analysis; Pollen Analysis; Preservation, Modes of; Starch Grain Analysis; Taphonomy; Vertebrate Analysis.

 World History
World History









