When ancient pottery is submitted to chemical analysis, the archaeological research questions must have been first defined: on provenance, technology, or usage. In order to guarantee the maximum quality of the obtained data, the collaboration between analytical chemists and archaeologists must also be encouraged and both must be involved in defining these questions, within the aim of a better understanding of the past.
In the analysis of archaeological materials, the problems facing the analytical chemist are in general quite different from those in the conventional analytical laboratory; therefore some particular requirements of analytical techniques must be considered, related to the type of analysis, the type of object, or the nature of analysis. In the case of pottery, the analysis can be specific to a single object or small groups of objects, but generally the analysis is systematic, on a large number of samples with a statistical significance. The heterogeneous nature of the pottery is an important feature of the material which influences the amount of sample taken for analysis, and also the object size or its specificity should be considered in the sampling or in the election of a nondestructive analytical method. The choice of the final method of analysis employed is related to the type of archaeological problem to be solved and the necessary analytical information to be extracted (see Sampling Methods, Theory and Praxis).
The chemical analysis of ceramics, also called elemental analysis, provides identification and quantification of the elements present in the samples. The results are given as weight percentage (wt.%) for major and minor elements or in units of microgram per gram (pgg-1, also called ppm, parts per million) for trace elements. In pottery analysis, major elements are expressed as element oxides, due to the clay nature, by means of the stoichiometric proportions of the oxide formula.
By chemical analysis, the elemental composition of pottery could be determined for major, minor, and trace elements. Ceramics are formed by clay materials with different quantities of nonplastic inclusions, natural or added as temper, so elements like silicon, aluminum, calcium, iron, potassium, magnesium, etc., appear in high proportions. The choice of what elements will be determined depends on the analytical information needed to solve the archaeological problem; in general, major and minor elements define more the nature of clays and the raw materials being used and trace or ultratrace elements are preferred for provenance studies. Generally, the chemical composition of the pottery is given as ‘bulk’ or total composition of the ceramic body, although sometimes only the ceramic surface is interesting to be analyzed.
Other factors that need to be considered in interpreting chemical composition data are possible changes in composition either during firing (very unlikely), or during burial subsequent to discard. Postdepositional geochemistry should be taken into account, especially if there was any groundwater around in the burial environment, and consequently only immobile elements should be considered in defining compositional groups.
In ceramic studies, the steps summarized in Figure 1 can be followed for chemical analysis in ancient material. Some of these steps are described in the next sections, especially those related to the object sampling and the selection of the analytical technique.
Sample Selection and Preparation
The chemist should assist in the selection of pottery for chemical analysis in order to ensure that the fragments studied are representative of the available assemblage. Except for specific analysis of precious objects, usually pottery analysis involves the selection of a group of fragments with statistical significance, whether we study provenance or research technology or usage. Because of the ceramic-body compositional heterogeneity (a term meaning that the body composition varies from one part to another) and the variability in chemical compositions within an individual raw material source and the possible similarity of different sources, ceramic studies based on chemical compositions involve the analysis of a large number of samples, which are then grouped together using statistical methods. In selecting ceramic fragments for analysis, it is important that, as far as is feasible, each group (ideally 15-20 samples) is made up of pottery of a single type, period, and site.
After the fragment or group of fragments has been selected, the first main question that should be asked is that it is possible to sample the objects. Here sampling means to separate a small part of the material being analyzed. In some cases for different reasons, no small part of the object can be taken to continue the chemical analysis, and then only a nondestructive analytical method can be chosen. But, if the object can be sampled, we can resort to a nondestructive or also to a destructive method. However, to be able to use a destructive method has the advantages, in general, of using more attainable and less expensive analytical techniques, avoiding surface compositional differences or contaminations, and, most importantly, being capable to sample a bigger quantity of ceramic and then obtaining a more representative analytical result of the object composition.
Archaeological
Data
Archaeological
Questions
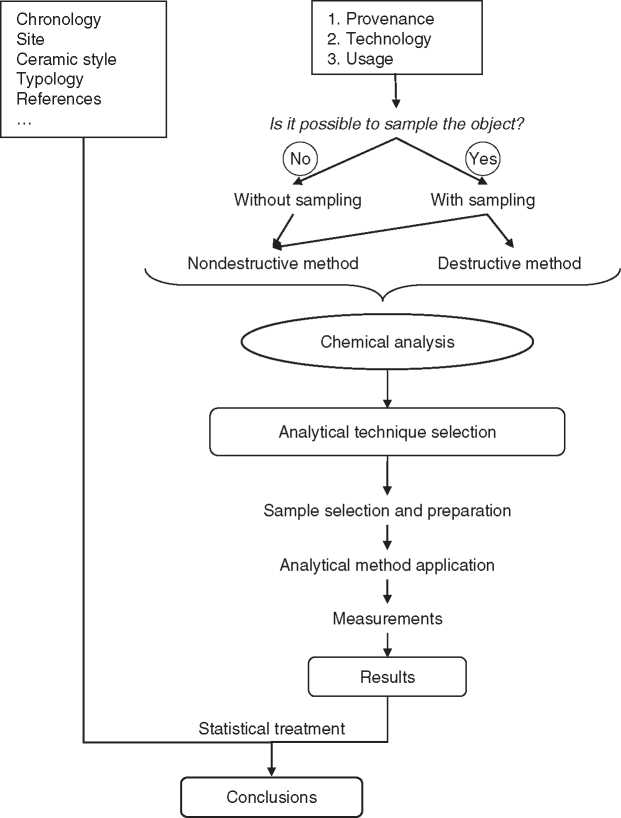
Figure 1 Steps in the chemical analysis of ancient pottery.
If we have decided to sample the objects (see Figure 2) and we want to perform a meaningful chemical analysis, first of all, we must obtain a small, homogeneous sample, whose composition is representative of the larger fragment, from each selected ceramic fragment. The small sample is obtained by drilling or by grinding to a fine powder and it is collected by taking portions from several places chosen at random because of the ceramic body heterogeneity.
Sample preparation should take into account that the variance of the sampling operation will be added to complete the overall variance, therefore all steps in this operation should contribute to reduce the uncertainty. This is why the particle size of the solid sample must be reduced as much as possible, and the sample size and the number of replicate analyses are decided according to a desired level of sampling variance with a specific confidence level.
After the solid sample is prepared, guaranteeing sample representativeness and avoiding contamination, the analytical procedure selected for analysis can be applied on the solid sample (i. e., on pressed pellets), but sometimes the sample needs to be dissolved and acid digestion or fusion may be used to convert it in a sample solution.
A large number of analytical techniques can be chosen for the chemical analysis of archaeological ceramics. The most popular analytical methods that have been used in pottery analysis are NAA, X-ray fluorescence (XRF) spectrometry, and inductively coupled plasma-atomic emission spectrometry (ICP-AES), or more recently inductively coupled plasma-mass spectrometry (ICP-MS).
Until the 1980s, the standard analytical method for multi-trace-element analysis was NAA, because of its detection limits and the possibility of a nondestructive analysis. NAA is a technique where some of the elements in the sample are converted into artificial radioactive elements by irradiation with neutrons. The artificial nuclei decay by one or several standard pathways for radioactive decay, and, if this radioactive decay is detected and the emission intensity measured, this is related back to the original concentration of the parent element in the irradiated sample. Routinely detection levels for NAA are of the order of 10ngg-1 or better, up to perhaps 10 mgg-1, although there is a variation in the sensitivity and detection limits from element to element, and not all the elements can be determined.
XRF is an analytical technique used for the determination of major and minor elements, with the advantage of analyzing directly the solid samples.
In XRF, X-rays used to irradiate the sample strike the solid and several processes take place, but one of them, the transference of part of the primary energy to the atoms, results in vacancies in the sample atoms because of the ejection of an electron. These vacancies give rise to a secondary (fluorescent) X-ray emission with characteristic wavelengths. This X-ray emission is the basis of the identification and quantification of the elemental composition of the sample. Two approaches are possible for the detection of the emitted X-ray: energy-dispersive X-ray spectrometry (EDXS) and wavelength-dispersive X-ray spectrometry (WDXS). In general, analytical performance of WDXS systems is better, with lower detection limits and higher precision.
The principal limitation on the analytical accuracy of those techniques (NAA and XRF) that required solid reference samples is the quality of the certified standard reference materials.
ICP-AES requires the sample to be in a liquid form (normally in an aqueous solution). The liquid sample is aspirated into the plasma and the high plasma temperature makes possible the dissociation of all compounds and they even attain an excited state, so that they strongly emit characteristic lines. These emission lines belong to the ultraviolet-visible (UV-Vis) region of the electromagnetic spectrum, and they are related to the presence and proportion of the chemical elements in the sample. ICP-AES allows a multielement analysis with low detection limits and good precision for major, minor, and trace elements in ceramic analysis.
A major benefit of the ICP is the possibility of being connected to an MS to give a more powerful technique: ICP-MS. Plasma temperature is high enough to ionize a high proportion of the sample atoms, making the plasma an ideal source of ions that can be extracted from the plasma and injected into a mass spectrometer. Individual charge ions are then separated according to their mass-charge ratio and they can be counted separately in order to be identified and quantified; this provides a chemical analysis of the sample. ICP-MS has the advantages of even lower detection limits and the possibility of monitoring the concentration of individual isotopes.
Some differences in analytical sensitivity, precision, and accuracy exist among techniques and laboratories involved in the chemical analysis of archaeological ceramics (see Chemical Analysis Techniques). Large differences in these analytical parameters become significant in the formulation of databases where comparability of the data is being wanted. Small differences become significant when comparing pottery produced from clay resources located within a discrete geological environment. But these differences do not prevent to obtain good analytical results.
In every case, the level of precision required for ceramic characterization studies, the use of standards, and the preparation and submission of samples for laboratory analysis should be evaluated in order to select the best analytical technique that allows the archaeological questions defined to be answered.
Finally, some statistical tools help us to present and to evaluate the analytical results obtained. Data are given as average values of several replicates, together with their standard deviation (as an estimation of the precision). Some statistical tests are useful to avoid errors and to evaluate accuracy. Student’s t is a statistical tool used most frequently to express confidence intervals (one of the most common estimations of the uncertainty in experimental measurements) and for comparing results from different experiments to decide whether they are ‘different’ or ‘the same’ from each other.
Most of the ceramic studies lead to a high number of data (many chemical components from numerous groups of samples); therefore statistical multivariate analysis of these data is necessary to understand the results. Some of the most usual statistical treatments are principal components analysis (PCA) or hierarchical cluster analysis (HCA), which help to reduce the number of variables and to group the samples.




 World History
World History









